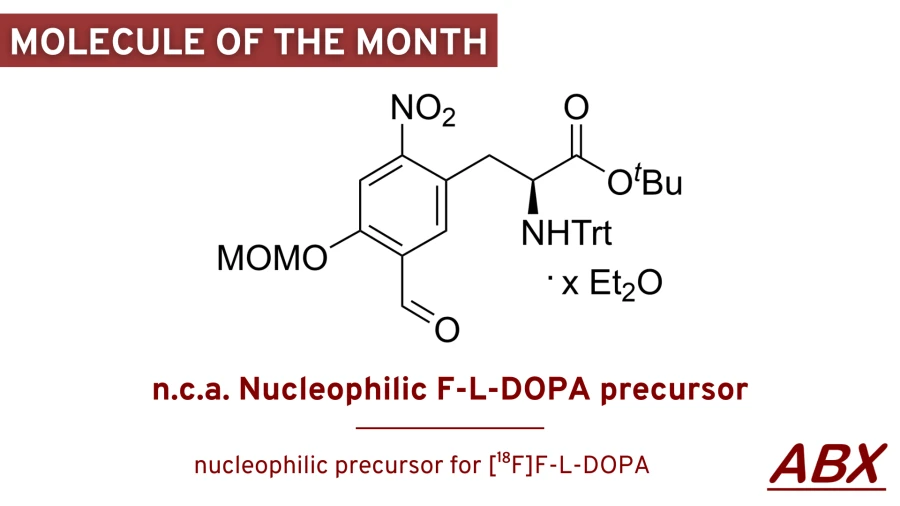
Welcome to our next molecule of the month 2024 - n.c.a. Nucleophilic F-L-DOPA precursor
The nucleophilic method for production of [18F]F-L-DOPA developed by Coenen et al. uses an isotopic [18F]-fluorine-[19F]fluorine exchange and provides [18F]F-L-DOPA in good yield but with low specific activity [1]. Based on the approach by Coenen et al., ABX developed a new precursor which allows the nucleophilic synthesis of [18F]F-L-DOPA using mild conditions suitable for a disposable kit [2]. The one-pot production with simple cartridge cleaning is a reliable and convenient method for routine clinical production. As the process allows the application of mild temperatures and reagents, only three minor by-products have been detected which are easily removed using SPE to finally provide a solution of [18F]-L-DOPA with high specific activity and enantiomeric purity.
Learn more about the related products and send us your request!
n.c.a. Nucleophilic F-L-DOPA precursor
Reference standard for 6-[¹⁸F]Fluoro-L-DOPA
Reagent Kits and Cassettes for the automated synthesis of 6-[¹⁸F]Fluoro-L-DOPA
[1] Wagner F. M. et al. Three-Step, “One-Pot” Radiosynthesis of 6-Fluoro-3,4-Dihydroxy-L-Phenylalanine by Isotopic Exchange. J. Nucl. Med. 2009, 50, 1724–1729.
[2] Martin R. et al. Automated nucleophilic one-pot synthesis of [18F]F-L-DOPA with high specific activity using the GE TRACERlab® MXFDG. J. Label. Compd. Radiopharm. 2013, 56, S126.
Learn more about the related products and send us your request!
n.c.a. Nucleophilic F-L-DOPA precursor
Reference standard for 6-[¹⁸F]Fluoro-L-DOPA
Reagent Kits and Cassettes for the automated synthesis of 6-[¹⁸F]Fluoro-L-DOPA
[1] Wagner F. M. et al. Three-Step, “One-Pot” Radiosynthesis of 6-Fluoro-3,4-Dihydroxy-L-Phenylalanine by Isotopic Exchange. J. Nucl. Med. 2009, 50, 1724–1729.
[2] Martin R. et al. Automated nucleophilic one-pot synthesis of [18F]F-L-DOPA with high specific activity using the GE TRACERlab® MXFDG. J. Label. Compd. Radiopharm. 2013, 56, S126.